This article was created to educate students on the basics of degenerative myopia. But really there is something deeper here. The authors felt that patients with high myopia really rely on their optometrist at yearly eye exams and these patients want to feel confident in their OD. Patients with high myopia can have vision threatening fundus pathology and it is important that as optometry students we really understand what is going on in their eyes. Expressing to your patients that you thoroughly understand their condition will help you to build a patient base when you are out in practice. There should be no need to refer their patients out. Understanding degenerative myopia and attracting these patients to your practice is a great way to ensure your future success as an OD.
BACKGROUND INFORMATION
Degenerative myopia is severe myopia greater than 6.00 Diopters that begins in youth (usually pre-teen years) and progresses in stages. To be classified as degenerative myopia, the myopia must be associated with degenerative changes in the posterior segment of the eye. Generally, myopia is the result of elongated axial length in the region of 25.5 or 26.5mm. (1,2)
Degenerative myopia is also known as other terminology including:
- High Myopia
- Degenerative Myopia
- Pernicious Myopia
- Malignant Myopia
EPIDEMIOLOGY
The epidemiology of this ocular condition generally has a prevalence with refractive error of greater than -7.9D. In the USA 0.2% – 0.4% of general population are classified as degenerative myopes while 1% of the Japanese population (approximately 1.25 million) are degenerative myopes. Overall, 6-18% of myopes meet the degenerative criterion. (3,4)
A very common question is how do patients become myopic? The answer is complex and involves many different mechanisms encompassing various systemic disorders, ocular disorders, and genetics:
- Inheritance
- Retinopathy of prematurity
- Marfan’s syndrome
- Ehler’s-Danlos syndrome
- Stickler’s syndrome
- Albinism
- Interruption of light passing through ocular media
- Genetics! autosomal dominant pathologic myopia to gene 18p11.31
FUNDUS SIGNS
It is important as primary eye care providers to be cognizant of the signs of degenerative myopia which commonly include:
- Tilting of the optic disc
- Peripapillary chorioretinal atrophy
- Vascular straightening
- Lacquer cracks
- Round, subretinal hemorrhages that clear spontaneously
- Fuchs spots + CNV
- Posterior staphyloma
- WSP
- Paving-stone degeneration and lattice degeneration
- Hole formation in the peripheral retina
- Elongation and atrophy of the cilliary body
- POAG
The question becomes, which of the following signs are most common in this population of degenerative myopes? 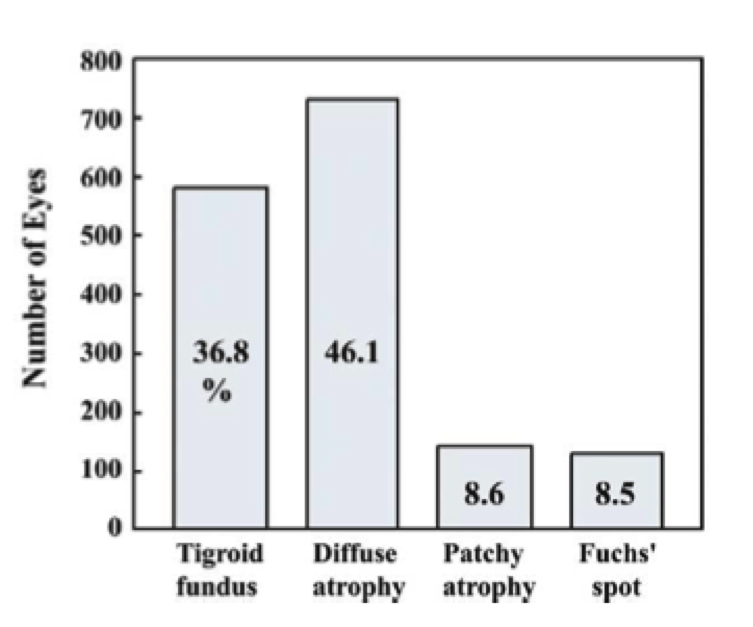 Several studies were conducted, and it appears that a majority of high myopes possess diffuse atrophy and fuchs spots.
Several studies were conducted, and it appears that a majority of high myopes possess diffuse atrophy and fuchs spots.
The graph on the top left shows the prevalence of fundus changes in 1,584 consecutive eyes with high myopia. The graph on the bottom right shows the VA log MAR with different fundus changes in 1,584 consecutive eyes with high myopia. (5)

TOOLS TO MONITOR
Once a patient has been established as a degenerative myope, what are the next steps? How do we manage and monitor these patients?
- Fundus photography
- A- and B-scan ultrasonography
- Visual fields
- OCT
- Optos photography
- Monitor corneal health if CL wearer
- Fundus photos
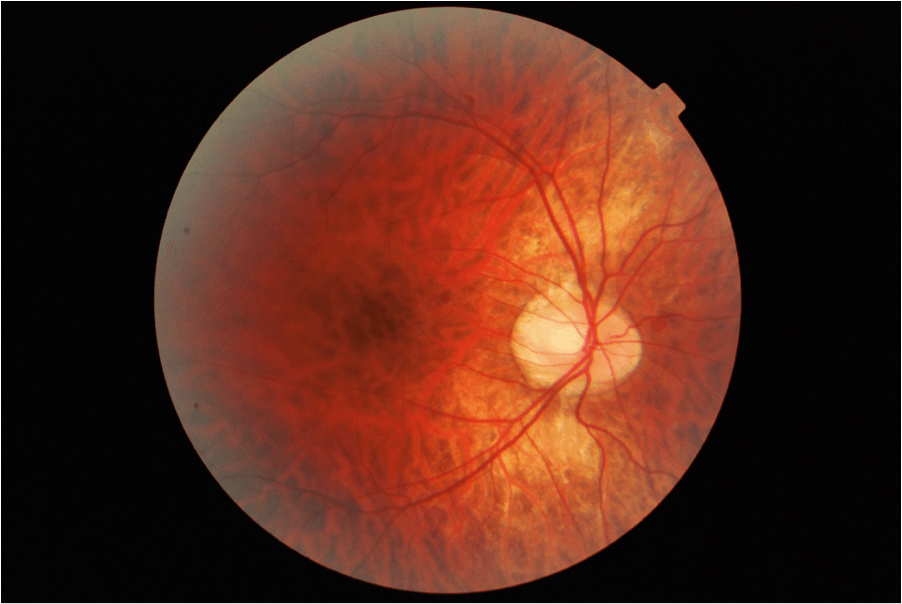
TIGROID FUNDUS [Photo Courtesy of Co-Author of this article: Antonio Chirumbolo]: As the eye enlarges, the retinal pigment epithelium thins, resulting in a tessellated (checkered) appearance of the fundus and increased visibility of the choroidal vasculature.
 POSTERIOR STAPHYLOMA: Staphylomas are localized ectasia (“enlargement”) of the sclera, choroid, and RPE. It can be easily seen on a B-scan or CT Scan. Staphylomas can eventually lead to atrophy and loss of vision.
POSTERIOR STAPHYLOMA: Staphylomas are localized ectasia (“enlargement”) of the sclera, choroid, and RPE. It can be easily seen on a B-scan or CT Scan. Staphylomas can eventually lead to atrophy and loss of vision.
 FUCHS SPOTS: Fuchs spots are dark spots due to RPE hyperplasia. They can involve subretinal neovascular membrane with an overlying retinal pigment epithelial hyperplasia. The CNV can eventually cause disciform scars on the macula in the 4th-6th decade of life.
FUCHS SPOTS: Fuchs spots are dark spots due to RPE hyperplasia. They can involve subretinal neovascular membrane with an overlying retinal pigment epithelial hyperplasia. The CNV can eventually cause disciform scars on the macula in the 4th-6th decade of life.
LACQUER CRACKS: Lacquer cracks are spontaneous ruptures of the elastic lamina of Bruch’s membrane that appear yellowish-white and are usually located in the posterior pole. They generally have linear or stellate  patters. IVFA will show hyperfluorescence as the fluorescein leaks through Bruch’s membrane, highlighting these cracks. These can lead to CNV in the 4th-6th decade of life.
patters. IVFA will show hyperfluorescence as the fluorescein leaks through Bruch’s membrane, highlighting these cracks. These can lead to CNV in the 4th-6th decade of life.
 LATTICE DEGENERATION: Lattice degeneration is a vitreo-retinal degeneration that causes retinal atrophy (“thinning”). It can be classified as pigmented or non pigmented. It takes on a lattice formation (“crisscrossing”) because the retinal vessels become sclerotic, and the collagen is laid down in this crisscross pattern. Due to the retinal thinning, it is prone to causing retinal breaks, tears, or holes, which could potentially lead to retinal detachment. However, it is important to remember that retinal breaks due to lattice degeneration rarely turn into retinal detachments.
LATTICE DEGENERATION: Lattice degeneration is a vitreo-retinal degeneration that causes retinal atrophy (“thinning”). It can be classified as pigmented or non pigmented. It takes on a lattice formation (“crisscrossing”) because the retinal vessels become sclerotic, and the collagen is laid down in this crisscross pattern. Due to the retinal thinning, it is prone to causing retinal breaks, tears, or holes, which could potentially lead to retinal detachment. However, it is important to remember that retinal breaks due to lattice degeneration rarely turn into retinal detachments.
LATTICE WITH ATROPHIC HOLES: Atrophic holes occur due to retinal thinning. Vitreous traction is not the pathogenic mechanism. Atrophic retinal holes are often associated with lattice degeneration. The incidence of retinal holes in lattice degeneration ranges from 18-42%. Atrophic holes are generally asymptomatic and rarely require treatment.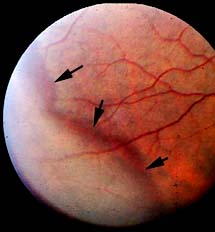
WHITE WITHOUT PRESSURE (WsP): WsP refers to geographic areas of apparent “whiteness” in the peripheral retina that is not caused by scleral indentation (therefore, without pressure). It is an optical phenomenon most likely related to collagen deposition at the retinal interface. In degenerative myopia, it is indicative of an area where the retina has been stretched, and is correlated with an increased risk for future retinal detachment in these eyes.
TILTED DISCS: Tilted discs are the result of oblique insertion of the optic nerves into the globe. The tilt is usually located inferonasal or inferotemporal. Corresponding arcuate 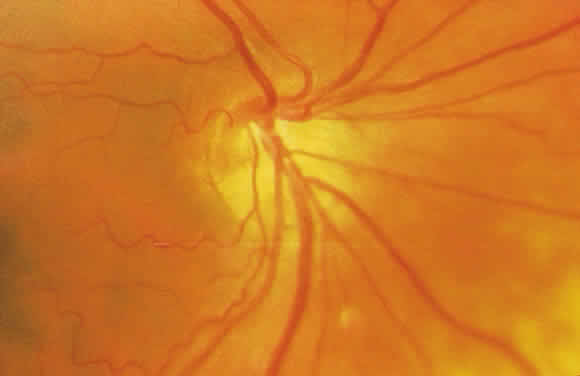 blood vessels are often directed nasally before sweeping temporally. This misdirection of vessels is termed situs inversus. Visual field loss is common in patients with tilted discs and often presents as a bitemporal hemianopic field loss, especially when a scleral crescent is present inferotemporally. Visual acuity can be mildly or moderately impaired and is often attributed to an overlying refractive amblyopia.
blood vessels are often directed nasally before sweeping temporally. This misdirection of vessels is termed situs inversus. Visual field loss is common in patients with tilted discs and often presents as a bitemporal hemianopic field loss, especially when a scleral crescent is present inferotemporally. Visual acuity can be mildly or moderately impaired and is often attributed to an overlying refractive amblyopia.
CHOROIDAL NEOVASCULARIZATION (CNV): CNV is the growth of new blood vessels that originate from the choroid through a break in Bruch’s membrane into the sub-retinal pigmented epithelium (sub-RPE) or subretinal space, creating what is called a choroidal neovascular net. These vessels subsequently break and cause fibroblast proliferation, a disciform scar, and loss of vision (if the macula is involved). Mechanisms of CNV are not well understood. Virtually any pathologic process that involves the RPE and damages Bruch’s membrane can be complicated by CNV. CNV may be considered as a wound healing response to an insult of the RPE. CNV is a major cause of visual loss.
Among all the myopic fundus pathologies, macular CNV is the most common vision-threatening complication reported to occur in 5-10% of patients with high myopia. (6)
The incidence of myopic CNV in patients with pre-existing myopic CNV in the fellow eye is even higher, as more than 30% of patients will develop CNV in the second eye within eight years after the first eye. (7)
Choroidal neovascular membranes are small, flat, subretinal membranes with a grey appearance in the fundus. They are typically less than 1 disc diameter in size and are located between the neurosensory retina and the RPE. The membrane forms in response to elevated VEGF and occurs primarily in the presence of lacquer cracks also associated with high myopia. Membranes are frequently subfoveal or juxtafoveal with minimal subretinal fluid or exudate. Patients with significantly high myopia must be monitored for membrane formation, which may warrant treatment with argon laser photocoagulation, anti-VEGF therapy, or photodynamic therapy if present. The incidence of myopic CNV in a patient with a pre-existing membrane in the fellow eye is increased. Nominally, it is estimated that 30% of patients will develop a membrane in the second eye within 8 years of occurrence in the first eye.
WHAT DOES EVIDENCE-BASED MEDICINE TELL US?
- OCT EVALUATION: OCT evaluation of 134 eyes with -8.00D or more of myopia revealed a 9% occurrence of foveal retinal detachment when a staphyloma was present. Conversely, 0% of eyes had a retinal detachment when a posterior staphyloma was absent. (8)
- LASIK: Another study conducted in Spain by Alió et. al. concluded that LASIK for myopia over 10D is a safe procedure with myopic regression that slows down over time. The authors also concluded that a high rate of best spectacle corrected visual acuity increased in long-term observation. 196 myopic eyes with mean spherical equivalent of (-13.95 D) were treated with myopic LASIK at the Instituto Oftalmológico de Alicante, Spain using the VISX 20/20 excimer laser. 42% of eyes were within -1.00 D, 61% of eyes were within -2.00 D, 27.5% eyes underwent re-treatments attributable to under correction and/or regression, 5% lost more than 2 lines of BCVA, 40% showed a postoperatively uncorrected visual acuity of 20/40 or better, and 1% with more than 15 D myopic correction developed corneal ectasia. (9)
- QUALITY OF LIFE: Quality of life is inevitably an issue in patients with degenerative myopia, as the refractive errors can be debilitating in terms of career, scholastic endeavors, cost, etc. A cross-sectional study of patients with pathological myopia and control subjects was conducted by Takashima et. al. The influence of the disease on the daily lives and quality of life of patients were evaluated using a self-rated questionnaire. With 52 questions in total, subjects such as shopping, salary, insomnia, and activities with friends were addressed, among others. It was concluded that the functional status of daily life and the quality of life of these patients was reduced. This reinforces the importance of optometrists in terms of intervention and management of the condition. (10)
- POAG: Primary open angle glaucoma in relation to degenerative myopia also has its place in scientific literature. Lee et. al. studied 515 patients with POAG for a minimum period of 5 years to investigate a possible correlation between POAG and progression of visual field loss. Patients diagnosed with POAG and myopia larger than -6.00D had a greater progression of visual field loss and accounted for 21.8% of the eyes involved in the study. Keep in mind, the correlation is not that high myopia led to glaucoma, but rather that patients with POAG and high myopia had a greater progression of visual field loss. The Beijing Eye Study supports this result as well. (11)
- IOL IMPLANT: Intraocular lens implants have been used to treat moderate to high myopia, and was evaluated in a clinical trial sponsored by the U.S. Food and Drug Administration. An implantable contact lens designed to vault anteriorly to the natural lens was investigated in 523 eyes with a range of 3.00D to 20.00D of myopia. Results indicated that 60.1% of eyes had a resultant BCVA of 20/20 or better. In addition, 92.5% of eyes had a resultant uncorrected visual acuity of 20/40 or better. These findings support the safety, efficacy, and predictability of the implantable contact lens to treat moderate to high myopia. (12)
- PEDIATRICS: Children 10 years of age or less were included in a retrospective record review and analysis of high myopia at SUNY Optometry between 1998 and 2001. Subjects included had a spherical equivalent of 6.00D or more of myopia. Of 178 patients in the study, 75.8% had amblyopia or reduced visual acuity, 31.5% had strabismus (eso or exo equal), and 35.4% had anisometropia. Lastly, 81.4% of the patients had high myopia in the absence of significant ocular or systemic conditions. (13)
- CONTACT LENSES: Lastly, contact lenses are widely used as a means of alleviating and correcting high levels of myopia. Advantages of this treatment modality include better cosmesis, reduced problems due to minification effects, less optical aberrations, less prism and vertex problems, and overall improvement in sporting activities. Disadvantages include a more costly aspheric or lenticular design, thicker modulus lens, possible lid and eye discomfort, and irritating lens movement.
In summation, high myopes are prevalent in our patient population and we should feel confident in managing such cases. By demonstrating your proficiency with this condition, patients will remain loyal, visit yearly, refer your services, and generate revenue. In turn, the ultimate reward is positively impacting their quality of life.
This article was written by Matthew Geller of SUNY Optometry and co-authored by Antonio Chirumbolo of SUNY Optometry and Stefania Paniccia of IAUPR Optometry. All students are graduating in the year 2013.
REFERENCED CLINICAL STUDIES
(1) Goss DA, Grosvenor TP, Keller JT, Marsh-Tootle W, Norton TT, Zadnik K. Optometric Clinical Practice Guideline Care of the Patient with Myopia. American Optometric Association, 1997. (http://www.aoa.org/documents/optometrists/CPG-15.pdf)
(2) Goss DA, Eskridge JB. Myopia. In: Amos JF, ed. Diagnosis and management in vision care. Boston: Butterworths, 1987:121-71.
(3) Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the US. Arch Opthalmol 1983;101:405-v407.
(4) Tokoro T. On the definition of pathological myopia in group studies. Acta Ophthalmol 1988;66:107-108.
(5) Tokoro T. Mechanism of axial elongation and chorioretinal atrophy in high myopia. Nippon Gannka Gakkai Zasshi 1994;98:1213-1237.
(6) Curtin BJ. Ocular findings and complications. In:Curtin BJ Editor. The myopias. Philadelphia: Harper & Row, 1985, pp 277-347.
(7) Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularization in pathologic myopia. Br J Ophthalmol 2003;87:570-3.
(8) Takayuki B, Kyoko OM, Soh F, Takeshi Y, Kenjiro Y, Ariko K, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. American Journal of Ophthalmology, 2003;135(3):338-342. (http://www.sciencedirect.com/science/article/pii/S0002939402019372)
(9) Alio JL, Muftuoglu O, Ortiz D, Pérez-Santonja JJ, Artola A, Ayala MJ, et al. Ten-year follow-up of Laser In Situ Keratomileusis for high myopia. American Journal of Ophthalmology 2008;145(1):55-64.e1. (http://www.sciencedirect.com/science/article/pii/S0002939407007817)
(10) Takashima T, Yokoyama T, Futagami S, Ohno-Matsui K, Tanaka H, Tokoro M, Mochizuki M. The quality of life in patients with pathologic myopia. Jpn J Ophthalmol 2001:45(1):84-92. (http://www.ncbi.nlm.nih.gov/pubmed/11163050)
(11) Lee YA, Shih YF, Lin LL, Huang JY, Wang TH. Association between high myopia and progression of visual field loss in primary open-angle glaucoma. J Formos Med Assoc 2008;107(12):952–957. doi: 10.1016/S0929-6646(09)60019-X
(12) Sanders DR, Vukich JA, Doney K, Gaston M. U.S. Food and Drug Administration clinical trial of the Implantable Contact Lens for moderate to high myopia. Implantable Contact Lens in Treatment of Myopia Study Group. Ophthalmology 2003;110(2):255-266. (http://www.ncbi.nlm.nih.gov/pubmed/12578765)
(13) Fitzgerald DE, Chung I, Krumholtz I. An analysis of high myopia in a pediatric population less than 10 years of age. Optometry. 2005;76(2):102–114.